CASE STUDY 03
LOUIS
Now that there are new opportunities for individualised treatments in healthcare, change is critical since processes and supply chains are turned upside down. Welcome to Louis, your dedicated pharmaceutical network for decentralised production. The platform facilitates healthcare services to effortlessly discover, license, and procure all the essential components required for personalised medication manufacturing, bringing healthcare closer to patients.
Impact (TLDR)
82%
of users felt the final design fully met their needs and said they would choose it over their current software
88%
SUS score achieved, indicating strong usability
31%
estimated reduction in user effort based on workflow analysis
The Problem
Digitised and heavily integrated over the past 20 years, supply chains of the healthcare system have become effective, but also fragile. Now as there are new opportunities for individualised treatments change is critical. Floods of new treatments to the individual patient are disrupting the industry as current supply chains are not equpped for these treatments.
Our Solution
A pharmaceutical network designed for decentralised production that brings the patients closer and connects all key players. This platform enables companies developing specialised medications to seamlessly discover, license, and procure all essential components for personalised, local manufacturing.

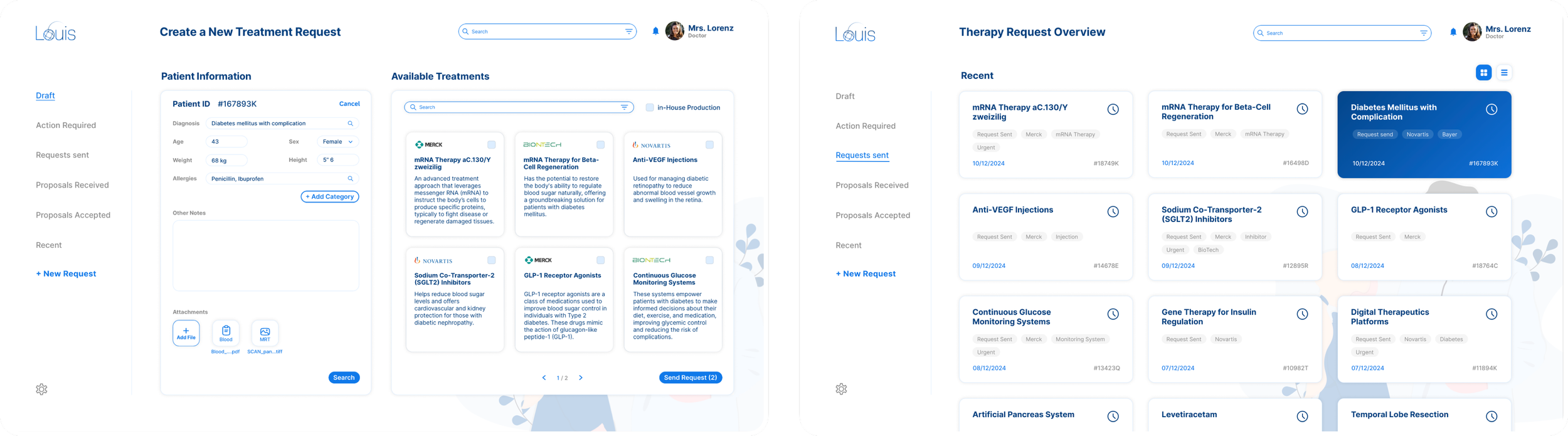
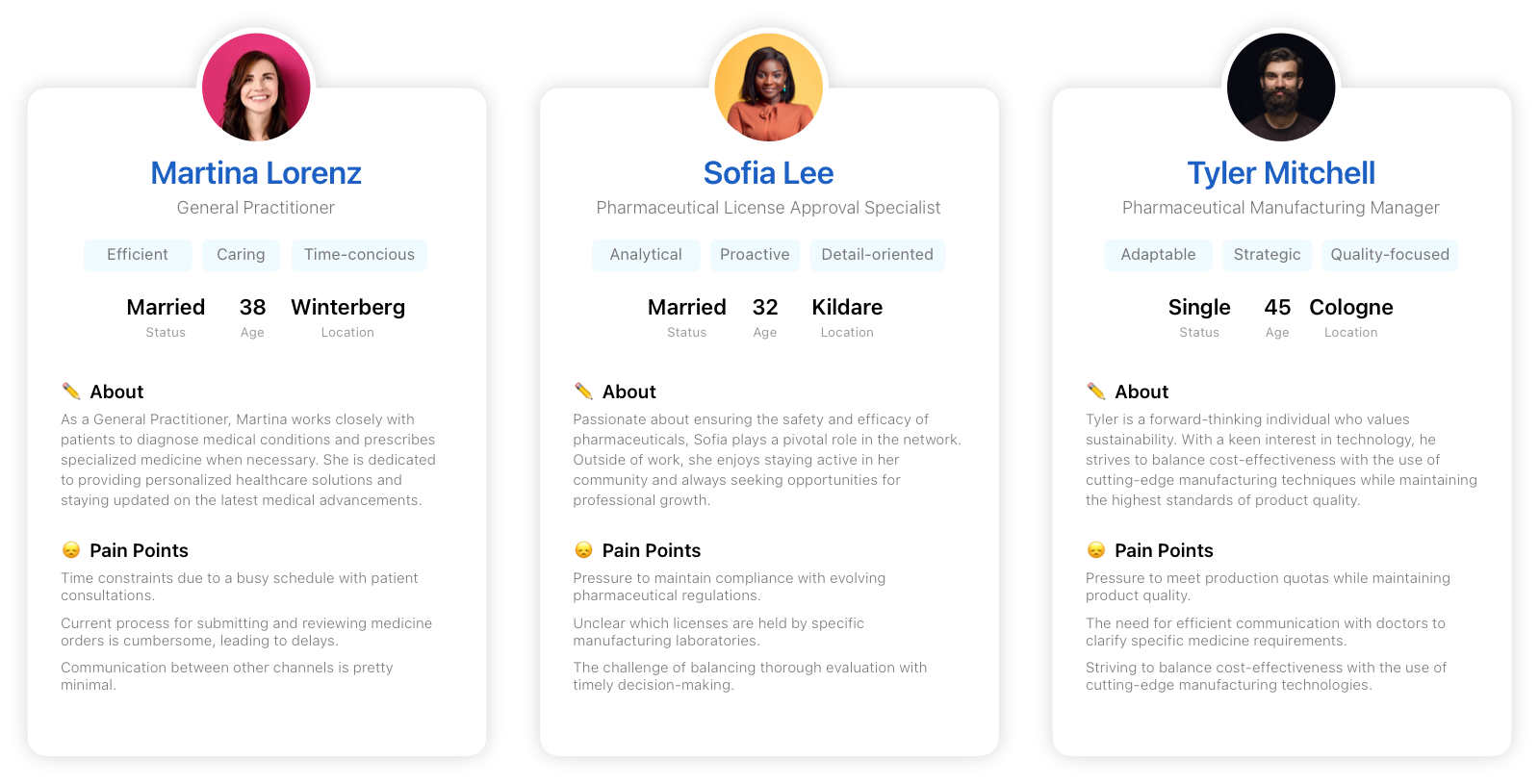
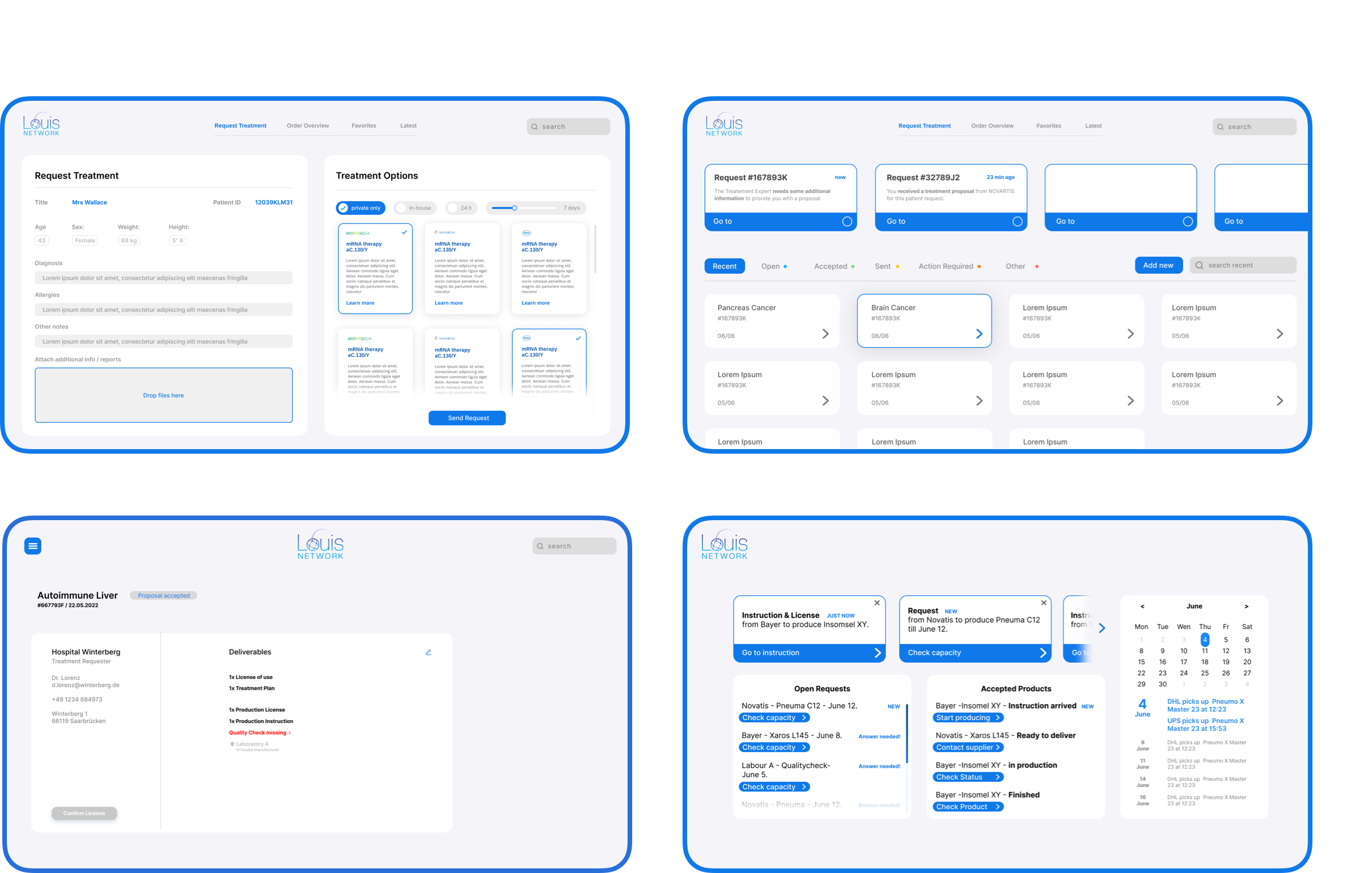
The Doctor
Using the LOUIS platform, doctors will be able to initiate treatment requests for patients when standard treatments are ineffective. Within this system, a variety of specialised treatment options will be made available to doctors, allowing them to submit orders for approval to the licensing department.
The License Provider
Upon a treatment request, the licensing provider can proceed to review and grant approvals for the use, production and quality and assurance licenses required for the specialised treatment to be manufactured for the patient. License providers are able to provide licenses to specific labs, improving the efficiency for the end patient.
The Manufacturer
Once the licenses have been granted to the doctor, the order will be sent on to the manufacturer based on the lab the doctor has selected. The manufacturer has their own dedicated dashboard through which they can oversee and manage production processes, document the production of treatments, and ensure adherence to established deadlines.

Our Process
The design thinking process we followed focused on encouraging idea exchange, ensuring team alignment, and gathering extensive insights from our users. We started by researching supply chains, consulting subject matter experts, and gaining a deep understanding of the target audience. This groundwork enabled us to craft user personas, need statements and journey maps that captured the user experience.
Following the research phase, we engaged in an iterative design process. Initially, we created design solutions individually, which were then refined and synthesised into cohesive final designs. This collaborative approach ensured that the final output aligned with user needs and project goals.
Preliminary Ideation
Before beginning the research phase, we focused on creating a collective understanding between all members in the team. We jotted down any assumptions, ideas that represented our knowledge of current pharmaceutical networks and any questions that needed to be answered later. It was fascinating to observe how these ideas evolved as we engaged with subject matter experts and deepened our knowledge in the field.
Competitive Analysis
We conducted a competitive analysis using the SWOT method to evaluate existing supply chain management software. This analysis helped us identify their strengths, weaknesses, and the opportunities for innovation within the industry, revealing areas where improvements and unique solutions can address unmet needs.
Interviews
Once we had outlined our assumptions and identified outstanding questions that required input from experts in the medical field, we proceeded to conduct interviews to understand the challenges faced by supply chain managers and validate our initial assumptions about their workflows and pain points.
We conducted three online interviews with subject matter experts. To guide these sessions, we prepared a discussion guide that included structured notes on how to navigate the interview, along with a list of questions and space for note-taking. To ensure consistency, we prioritized the questions that could be covered within the one-hour interview timeframe, noting any questions that were left unaddressed for future reference. This approach helped us maintain uniformity across all interviews.
Interview Results
The interviews provided a solid foundation for understanding the structural elements of supply chains, including distribution channels, manufacturing processes, and logistical intricacies, which greatly informed our design decisions later on. We synthesised the interview findings into three key categories:
The Not-So-Good
Understanding the pain points of our end users was crucial to ensure we were developing the right solution. A recurring theme across interviews was the growing prominence of specialised treatments and the acknowledgment that supply chains need improvements to enhance efficiency. This would enable patients to access treatments more quickly.
1. Specialised treatments often require intricate and time-consuming manufacturing methods.
2. Dependence on a few suppliers or distributors can create bottlenecks.
3. Fragmented systems across manufacturers, distributors, and healthcare providers
4. Lack of real-time data tracking to monitor inventory and transportation
5. Inefficient communication between stakeholders, leading to delays or errors
6. Specialised treatments are not easily obtainable for patients.
The Forgotten Party
Our initial plans and models identified two primary channels: the hospital and the manufacturer. However, through our interviews, we learned that this model was incomplete, as license providers play a crucial role in the distribution of medicine. One interview, in particular, highlighted the importance of specific licenses required for the production of specialised treatments, including licenses for use, production, and quality assurance.
Based on the insights we gathered, we refined our understanding and categorised the main channels involved in our pharmaceutical network as follows:
Need Statements
After conducting our interviews and further analysis of supply chains, we compiled a list of the significant issues affecting the individuals involved in this system that our network could potentially address.
Personas
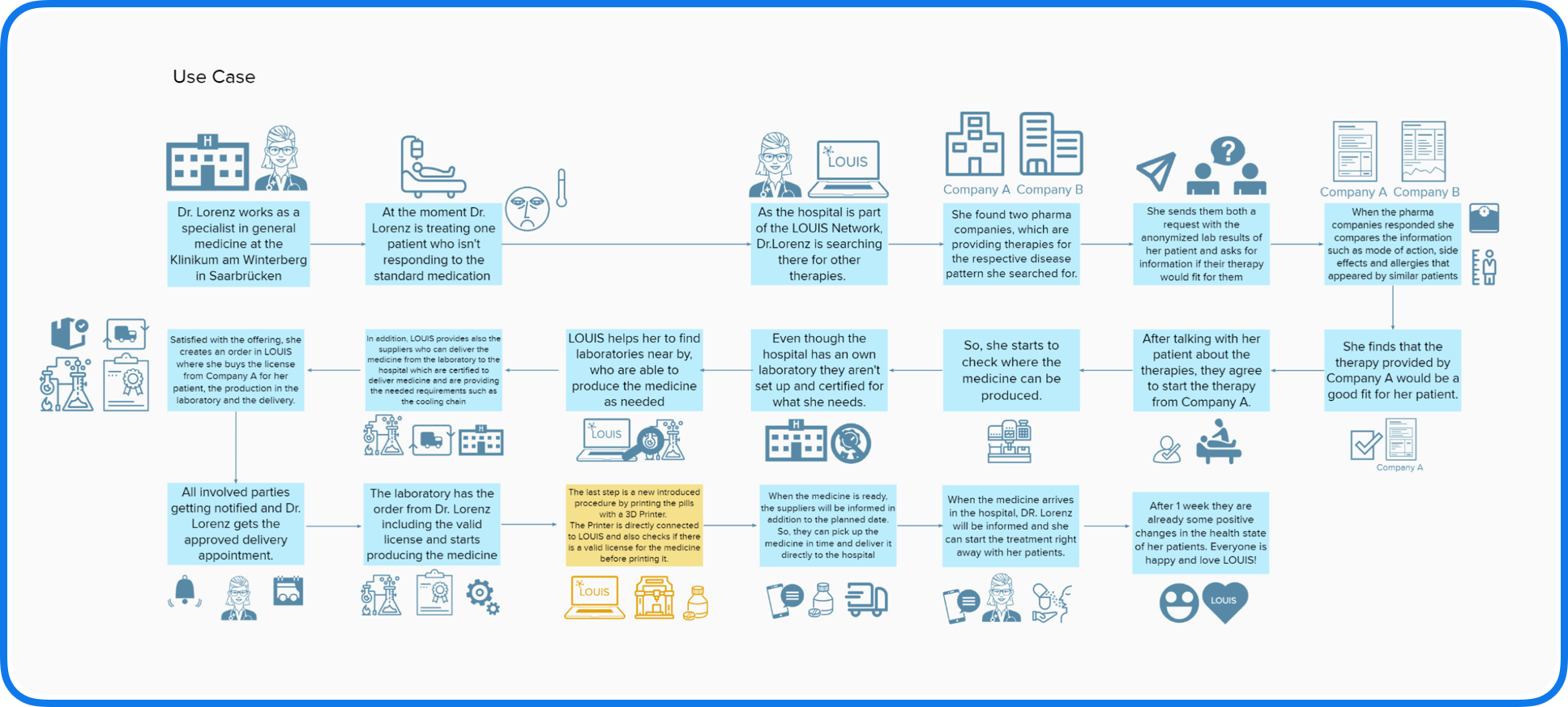
Use Case Diagram
After establishing our end users and need statements, our next objective was to visualise the interactions between the users and the network.
Information Architecture
We chose to utilise the use case diagram for a more detailed analysis, where we took notes outlining what the user would expect to see at each step. Additionally, we listed probing questions to guide our design decisions later in the process. This approach helped us identify the individual pages within each section of the application, which in turn defined the information architecture.
The Beginning of The Next Phase
With a clear understanding of our users, their pain points and their goals, we created a roadmap to guide us toward our goal of establishing a comprehensive design system and a fully functional prototype for the network. During this phase, our approach involved individual design work, followed by a collaborative review to identify and consolidate the most effective design elements before integrating them together into the final product.
Early Designs
During our first checkpoint, we presented the low fidelity designs we had created individually to stakeholders to gather feedback on what they liked and disliked. Each design had its own unique style and benefits, and we aimed to incorporate the strengths of each into a unified design. To achieve this, we held a team retrospective session to review the feedback and determine our next steps.
Uniting Our Design
For our second draft of low-fidelity wireframes, we focused on integrating the strongest elements from our initial designs. Some ideas were omitted, such as the web search idea, as our end users felt it was less effective compared to a design that allowed them to enter patient information and view a list of treatment options.
While creating the new draft, additional ideas emerged, including new formats and ways to present information. We conducted another design review to gather feedback, which we planned to incorporate into our high-fidelity wireframes.
Usability Test
When we were comfortable with our second draft of wireframes we mapped them into a simple prototype and asked our stakeholders to run through them and give us feedback. This proved to be some of the most benefitial feedback we would receive during the project as it allowed to get opinions on specific details that would make all of the difference to somebody using the application.
With this feedback we believed that we were ready to create high fidelity version of our design.
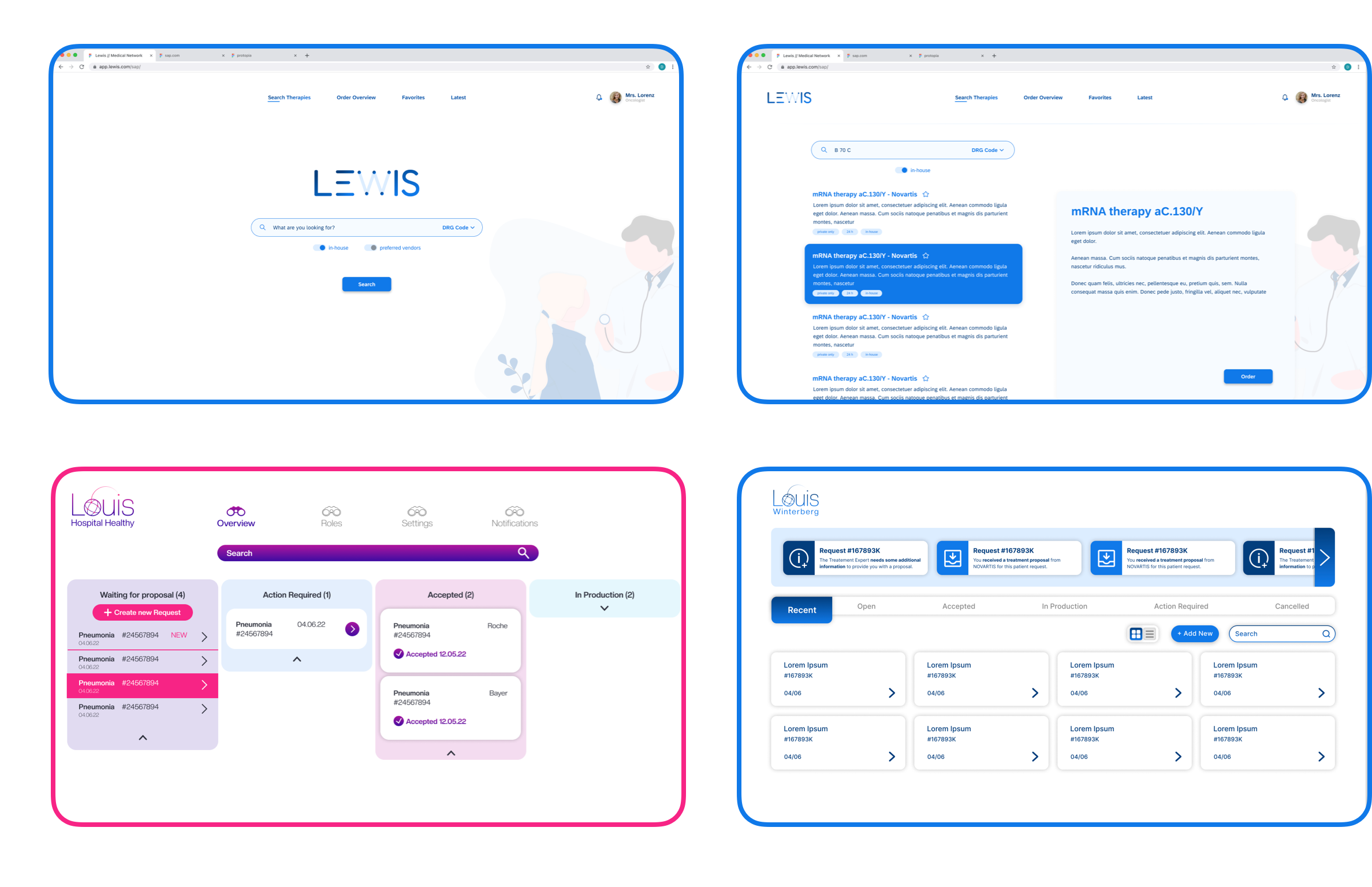
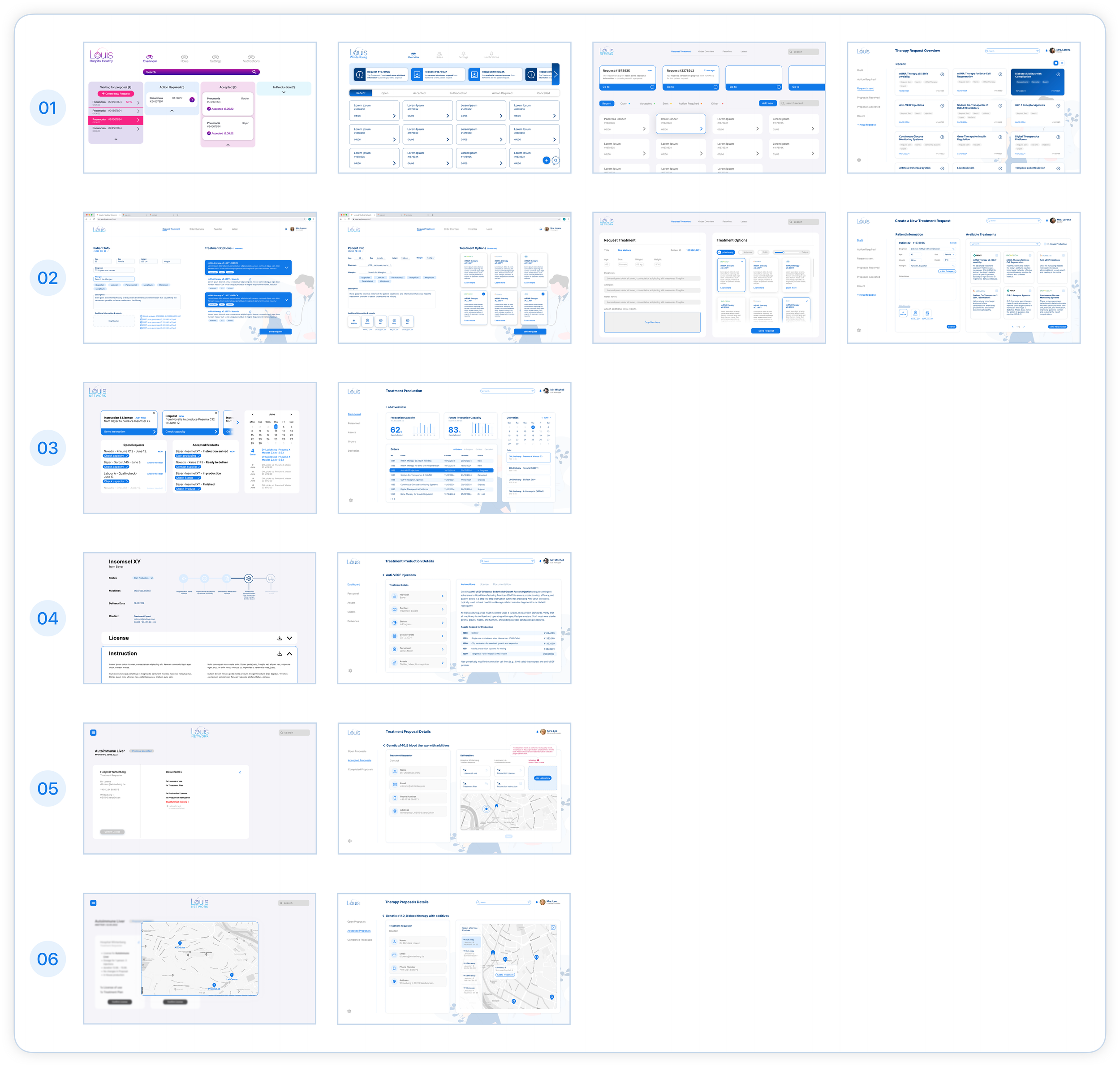
The Evolution of LOUIS
Challenges
Working on wireframes individually and then collaborating to merge the best ideas was a unique challenge I hadn't experienced before. Aligning on design decisions, such as the location of the tab bar, determining the layout of components on each screen, and finalising the color scheme, required effective communication and an open-minded approach to feedback.
Creating a tailored experience for users across three diverse industry sectors, which I was initially unfamiliar with, posed a significant challenge. It required extensive research and empathy to put myself in a position to create a solution for these users. While designing for a specific user, I considered how the design would adapt to the screens used by other users and how they would interact and communicate with one another.
Creating a Design System
In addition to developing an updated pharmaceutical network and high-fidelity wireframes, one of our key objectives was to establish a design system for this project.
We adopted atomic design as a methodology to break down design elements into smaller, reusable components, making our design process more systematic and efficient. This approach streamlined the reuse of components across the prototype, ensuring consistency in the design language throughout.

Takeaway
LOUIS is a concept that empowers users by granting them greater freedom in requesting specialised treatments, a particularly crucial development in a time when such practices are gaining popularity among patients. Teamwork and communication were essential in creating the final design, and the opportunity to collaborate with senior designers was invaluable. It enhanced my design process and broadened my perspective, highlighting new considerations to keep in mind when developing solutions in the future. If I had the chance to redo this project, I would focus on better organising the time allocated to the research phase. This would have allowed us to create more wireframes, particularly for the manufacturing and license approval processes, which would have resulted in a more detailed prototype.